|
Case Report
Renal artery embolization in a case of renal cell carcinoma with intractable hematuria with acute myocardial infarction
1 Consultant Urologist, Department of Urology, Rainbow Hospital, Agra, Uttar Pradesh, India
2 Chairman, Department of Urology and Renal Sciences, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh, India
3 Department of Urology and Renal Transplant, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh, India
4 Senior Consultant, Department of Urology and Renal Transplant, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh, India
5 Head of Department, Department of Urology and Renal Transplant, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh, India
6 Senior Resident, Department of Urology and Renal Transplant, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh, India
Address correspondence to:
Madhu Sudan Agrawal
Chairman, Department of Urology and Renal Sciences, Pushpanjali Hospital and Research Centre, Agra, Uttar Pradesh,
India
Message to Corresponding Author
Article ID: 100044Z15LS2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Sharma LK, Agrawal MS, Kumar N, Yadav A, Mishra D, Sharma G. Renal artery embolization in a case of renal cell carcinoma with intractable hematuria with acute myocardial infarction. J Case Rep Images Urol 2024;9(1):22–28.ABSTRACT
Introduction: Renal artery embolization is a valuable treatment option for various renal tumors, both malignant and benign. The indications for this procedure include preoperative arterial embolization of large and vascular renal cell carcinoma to reduce intraoperative hemorrhagic complications, management of malignant renal tumors in patients who are not suitable candidates for surgery, treatment of symptomatic hematuria and palliative care for metastatic renal cancer.
Case Report: In this case report, we present the case of a 56-year-old female with a known history of hypertension and cerebrovascular accident, who presented with complaints of hematuria and urinary retention. A diagnosis of left renal mass suggestive of renal cell carcinoma was made. The patient was planned for left radical nephrectomy and advised to discontinue aspirin for a week. However, on the morning of surgery, she experienced acute myocardial infarction and had to be hospitalized in intensive cardiac care. Aspirin was restarted, leading to recurrent severe hematuria. The patient underwent renal artery embolization, which successfully controlled the hematuria. Following cardiac stabilization, radical nephrectomy was performed three months later. The surgery and post-operative period were uneventful.
Conclusion: This case study demonstrates the use of renal artery embolization as an effective modality for controlling hematuria, and temporizing management in a case of renal cell carcinoma where surgery was not immediately feasible due to a medical emergency.
Keywords: Hematuria, Renal artery embolization, Renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is the most common malignant neoplasm of the kidney, predominantly composed of clear cells and often associated with von Hippel–Lindau syndrome [1]. Surgical resection remains the primary treatment for RCC, but the presence of extensive tumor invasion into the renal pelvis, renal vein, and inferior vena cava can complicate the surgical approach [1].
Renal artery embolization (RAE) has emerged as an effective minimally invasive alternative and palliative procedure for the treatment of renal tumors, both malignant and benign [2]. Since its development in the 1970s, RAE has evolved with technical advancements and increased experience, expanding its indications in various clinical scenarios [2]. The applications of RAE include preoperative treatment of RCC to decrease intraoperative hemorrhagic complications, management of malignant renal tumors in patients who are not suitable candidates for surgery, treatment of symptomatic hematuria, and palliation for metastatic renal cancer [3].
Renal artery embolization also plays a significant role in the management of other conditions at risk of spontaneous hemorrhage, such as angiomyolipoma, vascular malformations, medical renal disease, and complications following renal transplantation [4]. Additionally, RAE in metastatic renal cancer may stimulate tumor-specific antibodies, although the clinical benefits in terms of response or survival are not well-established [5].
In cases of large and complex renal masses with extensive vascularity and rapid local invasion, radical nephrectomy can be challenging for surgeons [6]. Preoperative embolization of RCC can improve surgical outcomes and reduce mortality rates compared to surgical treatment alone.
Renal artery embolization is generally well-tolerated with minimal complications, especially when the time interval between embolization and surgery is less than 48 hours [7]. Furthermore, literature supports the efficacy of RAE in providing palliation of symptoms, such as hematuria, in patients with inoperable advanced-stage renal cell carcinoma or those who are unfit for surgery [8].
In this case report, we present a clinical scenario of a patient with a diagnosed kidney cancer who developed massive hematuria while on antiplatelet therapy following an acute myocardial infarction on the morning of scheduled surgery.
Case Report
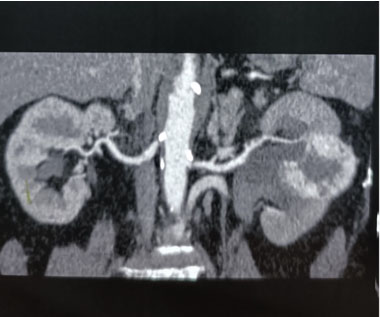
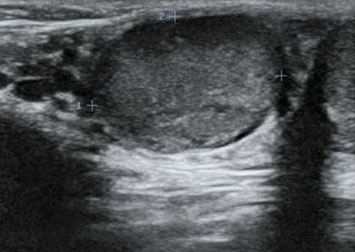
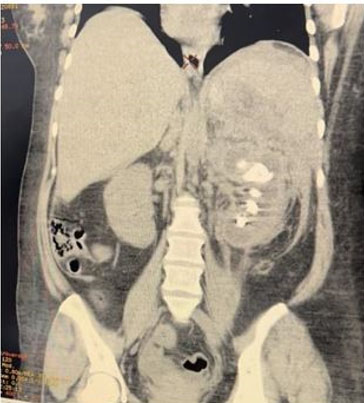
A 56-year-old female with a known case of hypertension and a history of cerebrovascular accident 12 years ago, on low-dose aspirin, presented with complaints of hematuria and clot retention for one day. Physical examination revealed a soft, non-tender abdomen with no palpable mass, but suprapubic fullness was present. Further investigation through abdominal ultrasound showed an iso- to hypo-echoic lesion with mild internal vascularity in the mid-pole region of the left kidney, measuring 43×42×31 mm, along with mild left-sided hydronephrosis. Additionally, a blood clot measuring 68×60×44 mm was found in the urinary bladder. A bladder wash was performed, and a three-way 18 Fr Foley’s catheter was inserted. Triphasic abdominal computed tomography (CT) scan revealed a 4.8 × 4.2 cm mass lesion in the mid-pole cortex of the left kidney, showing arterial enhancement, central low attenuation areas, and relative washout on delayed images (Figure 1). The lesion appeared partly exophytic, extending into the paranephric space and abutting the pelvicalyceal system. The renal artery and venous system appeared patent, without evidence of venous thrombus or peri-renal/retroperitoneal lymphadenopathy. Additionally, a 7 mm calculus was noted in the left mid-ureter with proximal ureteral dilatation (Figure 2).
The patient was diagnosed with a left renal mass, suspected to be renal cell carcinoma, and was planned for left radical nephrectomy after obtaining medical fitness. As part of the preoperative preparation, the patient was advised to discontinue aspirin for a week before surgery. However, on the morning of the scheduled surgery, the patient experienced chest pain. The electrocardiogram (ECG) changes were indicative of acute coronary ischemia. Cardiac markers confirmed an acute inferior wall myocardial infarction (MI). The patient was transferred to the intensive cardiac care unit and managed conservatively. As advised by the cardiologist, aspirin was restarted. During the hospital stay, the patient developed significant hematuria, resulting in severe anaemia that required the transfusion of 2 units of packed red blood cells. Due to the cardiac instability nephrectomy was deferred. The patient was prescribed Ecospirin 75 mg along with antihypertensive medications, and all potential risks were explained.
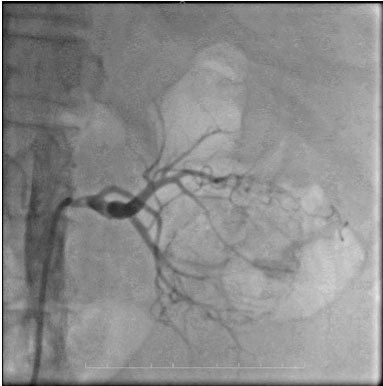
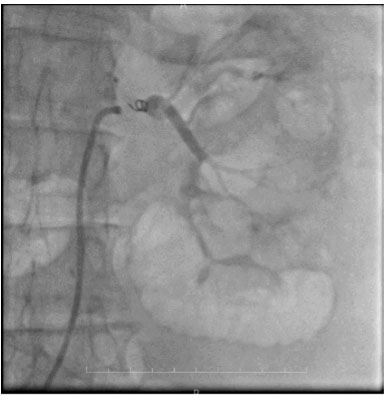
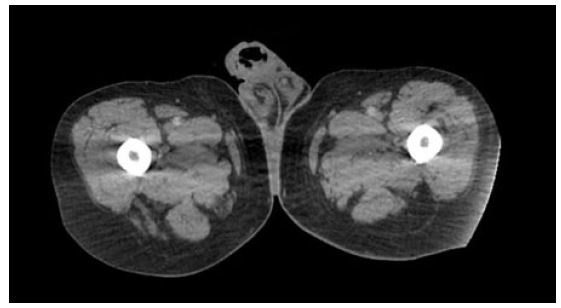
During follow-up, the patient experienced recurrent episodes of hematuria, requiring additional packed red blood cell transfusion. Due to the patient’s cardiac condition, she was deemed unfit for surgery, and conservative management was continued. The patient’s caregivers were counseled regarding the necessity of RAE for hematuria control and the subsequent delayed radical nephrectomy. Under the care of the cardiologist, renal artery angioembolization was performed using endovascular coil placement (Figure 3). The procedure was uneventful, and the patient was transferred to the urological ward after stabilization. Hematuria was immediately controlled following the procedure.
In the post-procedure period, the patient experienced malaise, leucocytosis, and a slight increase in serum creatinine, which were attributed to post-infarction syndrome and managed conservatively with analgesics, antibiotics, and antipyretics. On ultrasound examination of the abdomen in the follow-up period, the renal mass originating from the mid-pole of the left kidney measured 4738 mm, and showed heterogeneous soft tissue echo texture. The lesion displayed a few hypoechoic areas suggestive of necrosis, along with focal calcifications and vascularity. There were no complaints of hematuria during follow-up.
Two months after RAE, and three months after the acute MI, left radical nephrectomy was performed under general anaesthesia. Antiplatelet medications were discontinued five days prior to surgery. Intraoperatively, dilated collaterals entering the kidney and loss of fat planes were observed. The ureteric calculus was also removed. The surgery proceeded uneventfully, and the specimen was sent for histopathological examination.
The postoperative period was uneventful. The drain was removed on postoperative day 3, aspirin was restarted on postoperative day 5, and the patient was discharged on postoperative day 7. The patient remained stable in the follow-up with good renal functions.
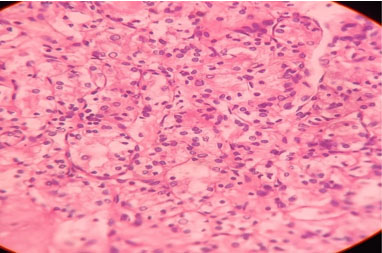
The cut section of the specimen revealed a partially necrotic exophytic mass in the central portion of the kidney. The histopathology report showed clear cell RCC (Figure 4).
Discussion
Hematuria can have various etiologies, including iatrogenic, traumatic, neoplastic, or spontaneous causes. While conservative treatment is often sufficient for self-limiting bleeding states, massive or continuous hematuria may require intervention to control the bleeding. In such cases, selective RAE is a viable therapeutic option [9],[10].
Renal cell carcinoma is the most common type of primary renal malignancy in adults, accounting for approximately 85% of cases. It primarily affects individuals over the age of 40, with a higher incidence in males. Certain conditions, such as von Hippel–Lindau disease, horseshoe kidneys, or polycystic kidney disease, may increase the risk of RCC. Hematuria is a common presenting symptom, present in about 50–60% of cases. Other signs include palpable tumors, abdominal or flank pain, but the classic triad of hematuria, renal mass, and flank pain is uncommon at the time of diagnosis now [11].
Patients with RCC who are on antiplatelet therapy are at a higher risk of experiencing troublesome episodes of massive or continuous hematuria. Emergency nephrectomy carries the potential for surgical complications, postoperative issues, and loss of renal reserve due to the aggressive removal of functioning renal tissue [12]. In such cases, RAE provides an alternative treatment option with low morbidity and is feasible even in patients with a low performance status.
Renal artery embolization has been used since 1964 and has undergone modifications over time, leading to the development of super-selective RAE techniques that minimize tissue loss [12]. The choice of embolic agents plays a role in the clinical outcome, including the cessation of bleeding and the likelihood of recurrence. Temporary agents such as gel foam, thrombin, and collagen granules, as well as permanent agents like coils, N-butyl cyanoacrylate (NBCA)-glue, polyvinyl alcohol (PVA) particles, and microvascular plugs (MVP), are commonly used for RAE [13],[14].
The technique of embolizing hyper vascular renal carcinomas was first reported in 1969 by Lalli et al. Since then, various techniques and embolic materials have been described. Renal artery embolization can be used preoperatively to facilitate nephrectomy or to stimulate a systemic response in patients with metastases. It is also an established palliative treatment option for unresectable renal carcinoma and patients with less advanced disease who are unsuitable for or unwilling to undergo surgery. The procedure reduces tumor bulk and provides relief from local symptoms, such as pain or intractable hematuria [15],[16],[17],[18].
In addition to its primary therapeutic benefits, renal artery embolization has been proposed to have immunological advantages by augmenting natural killer cell and lymphoproliferative responses triggered by the release of necrosis factors, thereby stimulating the immune response.
Our patient presented with a diagnosed renal mass while on antiplatelet therapy, and subsequently experienced an acute myocardial infarction on cessation of antiplatelet therapy, just before the planned operation. Due to the myocardial infarction, surgery had to be deferred, and antiplatelet therapy restarted. However, the delay in surgery raised concerns regarding the potential morbidity associated with continued hematuria and disease progression.
Renal artery embolization proved to be an effective minimally invasive method for controlling hematuria in our patient on antiplatelet therapy due to acute myocardial infarction. In the event of uncontrolled bleeding originating from a malignant renal mass, embolization is a safe and effective approach. It also provided temporary reprieve until the patient recovered enough to be fit to undergo definitive surgery for the renal tumor, thus serving a dual purpose.
Conclusion
This case study demonstrates the use of renal artery embolization as an effective modality for controlling hematuria, and temporizing management in a case of renal cell carcinoma where surgery was not immediately feasible due to a medical emergency.
REFERENCES
2.
Sauk S, Zuckerman DA. Renal artery embolization. Semin Intervent Radiol 2011;28(4):396–406. [CrossRef]
[Pubmed]

3.
Li D, Pua BB, Madoff DC. Role of embolization in the treatment of renal masses. Semin Intervent Radiol 2014;31(1):70–81. [CrossRef]
[Pubmed]

4.
Davis C, Boyett T, Caridi J. Renal artery embolization: Application and success in patients with renal cell carcinoma and angiomyolipoma. Semin Intervent Radiol 2007;24(1):111–6. [CrossRef]
[Pubmed]

5.
Kalman D, Varenhorst E. The role of arterial embolization in renal cell carcinoma. Scand J Urol Nephrol 1999;33(3):162–70. [CrossRef]
[Pubmed]

6.
Cochetti G, Zingaro MD, Boni A, et al. Renal artery embolization before radical nephrectomy for complex renal tumour: Which are the true advantages? Open Med (Wars) 2019;14:797–804. [CrossRef]
[Pubmed]

7.
Maxwell NJ, Saleem Amer N, Rogers E, Kiely D, Sweeney P, Brady AP. Renal artery embolisation in the palliative treatment of renal carcinoma. Br J Radiol 2007;80(950):96–102. [CrossRef]
[Pubmed]

8.
Sommer CM, Stampfl U, Bellemann N, et al. Patients with life-threatening arterial renal hemorrhage: CT angiography and catheter angiography with subsequent superselective embolization. Cardiovasc Intervent Radiol 2010;33(3):498–508. [CrossRef]
[Pubmed]

9.
Zugazagoitia J, Sastre J, Barrera J, García B, Díaz-Rubio E. Severe perirenal hematoma in a patient with a single kidney treated with sunitinib for metastatic pancreatic neuroendocrine tumor. J Cancer Res Ther 2012;8(2):303–5. [CrossRef]
[Pubmed]

10.
Springer C, Hoda MR, Fajkovic H, et al. Laparoscopic vs open partial nephrectomy for T1 renal tumours: Evaluation of long-term oncological and functional outcomes in 340 patients. BJU Int 2013;111(2):281–8. [CrossRef]
[Pubmed]

11.
Keeling AN, McGrath FP, Thornton J, Brennan P, Lee MJ. Emergency percutaneous transcatheter embolisation of acute arterial haemorrhage. Ir J Med Sci 2010;179(3):385–91. [CrossRef]
[Pubmed]

12.
Koganemaru M, Abe T, Iwamoto R, et al. Ultraselective arterial embolization of vasa recta using 1.7-French microcatheter with small-sized detachable coils in acute colonic hemorrhage after failed endoscopic treatment. AJR Am J Roentgenol 2012;198(4):W370–2. [CrossRef]
[Pubmed]

13.
Sam K, Gahide G, Soulez G, et al. Percutaneous embolization of iatrogenic arterial kidney injuries: Safety, efficacy, and impact on blood pressure and renal function. J Vasc Interv Radiol 2011;22(11):1563–8. [CrossRef]
[Pubmed]

14.
Lalli AF, Peterson N, Bookstein JJ. Roentgen-guided infarctions of kidneys and lungs. A potential therapeutic technic. Radiology 1969;93(2):434–5. [CrossRef]
[Pubmed]

15.
Kaisary AV, Williams G, Riddle PR. The role of preoperative embolization in renal cell carcinoma. J Urol 1984;131(4):641–6. [CrossRef]
[Pubmed]

16.
Nakano H, Nihira H, Toge T. Treatment of renal cancer patients by transcatheter embolization and its effects on lymphocyte proliferative responses. J Urol 1983;130(1):24–7. [CrossRef]
[Pubmed]

17.
Nurmi M, Satokari K, Puntala P. Renal artery embolization in the palliative treatment of renal adenocarcinoma. Scand J Urol Nephrol 1987;21(2):93–6. [CrossRef]
[Pubmed]

18.
Park JH, Kim SH, Han JK, Chung JW, Han MC. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodized oil. Cardiovasc Intervent Radiol 1994;17(6):323–7. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Laxmi Kant Sharma - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Madhu Sudan Agrawal - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Naveen Kumar - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anurag Yadav - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dilip Mishra - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gaurav Sharma - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Laxmi Kant Sharma et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.