|
Case Report
Septic shock secondary to xanthogranulomatous pyelonephritis: A case report and literature review
1 MD, General surgeon and laparoscopist, Division of General Surgery/Laparoscopy, Hospital General de Ciudad Juarez, Chihuahua, Mexico
2 MD, Urology surgeon, Division of Urology, Hospital General de Ciudad Juarez, Chihuahua, Mexico
3 MD, General surgeon and laparoscopist, Division of General Surgery/Laparoscopy, Centro Medico De Especialidades, Chihuahua, Mexico
4 MD, General surgeon resident, Division of General Surgery/Laparoscopy, Hospital General de Ciudad Juarez, Chihuahua, Mexico
Address correspondence to:
Cesar Alberto Lopez Jaime
Avenida Paseo Triunfo de la Republica, 32340 Ciudad Juárez, Chihuahua,
Mexico
Message to Corresponding Author
Article ID: 100042Z15CJ2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Lopez Jaime CA, Alonso Morales A, Perzabal Avilez CT, Hernandez Garza FN. Septic shock secondary to xanthogranulomatous pyelonephritis: A case report and literature review. J Case Rep Images Urol 2024;9(1):12–17.ABSTRACT
Introduction: Xanthogranulomatous pyelonephritis is described by histopathology as a destruction of the renal parenchyma and replaced by a chronic infiltrate of lipid-laden macrophages. It is known that patients with xanthogranulomatous pyelonephritis have a high rate of perioperative and postoperative morbimortality; however, there are few case reports on this entity.
Case Report: A 35-year-old woman attended the emergency department due to the presence of pain in the left flank, accompanied by an altered state of consciousness and respiratory distress. The study protocol started, identifying a significant increase in the left kidney, the presence of a central staghorn stone, dilated minor calices, and clinically with septic shock. The clinical conditions improved by adding intravenous fluids, vasoactive amines, red blood cells concentrates, and broad-spectrum antibiotics. Subsequently, a left nephrectomy was performed. On the following days, the septic shock gradually resolved, leading to the patient’s discharge. Eventually, histopathology report was reviewed, confirming a xanthogranulomatous pyelonephritis.
Conclusion: When a xanthogranulomatous pyelonephritis clinical suspicion is found, it is considered a surgical emergency. Furthermore, if proper management is not given immediately, there is a high risk of complications and death. However, the current statistics in Mexico have not been updated to contrast these data.
Keywords: Septic shock, Staghornn lithiasis, Xanthogranulomatous pyelonephritis
Introduction
Xanthogranulomatous pyelonephritis is a type of chronic pyelonephritis with a very low incidence of 0.6–1.4% of all chronic pyelonephritis [1]. It is described by histopathology as the destruction of the renal parenchyma and replaced by a chronic infiltrate of lipid-laden macrophages [1],[2]. In most cases xanthogranulomatous pyelonephritides are unilateral with renal obstruction due to calculus and granulomatous inflammatory state resulting in chronic inflammation, destruction of the parenchyma, and a hydronephrotic state, concluding in a non-functional kidney [3].
It was first described in 1916 by Schlagenhaufer and this condition occurs in approximately 1% of all kidney infections [4],[5].
The age range is about 50–70 years with a 2:1 woman:man ratio, with an incidence of 1.4 per 100,000 people/year. This suffering is potentially fatal and perioperative mortality has been reported to be as high as 40%, with at least 15% requiring admission to intensive care [6].
The microorganisms mostly found in urine cultures are Proteus mirabilis, followed by Escherichia coli, resulting positive in 60% of all urine cultures [7]. The stasis secondary to chronic renal lithiasis, repeated chronic infections, and an abnormal association with lipid metabolism or an abnormal immune reaction have been considered as possible etiological factors in xanthogranulomatous pyelonephritis, but there is still no experimental evidence to support this theory [7].
Its symptoms are not specific, it is associated with nephrolithiasis with urinary tract obstruction, urinary stasis, and an enlarged renal mass that is palpable on physical examination. The most frequent symptom is flank pain (92.5%), followed by fever (62.5%), dysuria (47.5%), and a palpable mass in the abdomen (30%) [8].
Computed tomography (CT) is considered the standard procedure, it allows to determine the magnitude of the involvement of the renal parenchyma and extra renal extension [7]. The classic radiological triad associates renal hypertrophy (enlarged kidney), central staghorn stone, and non-functional kidney [9].
The treatment of choice is surgery, managed mainly with nephrectomy with extensive debridement of necrotic tissue [1]. Nephrectomy in the context of xanthogranulomatous pyelonephritis has a high morbidity rate [1],[10].
Case Report
A 35-year-old woman attended the emergency department due to the presence of oppressive pain in the left flank, accompanied by an altered state of consciousness and respiratory distress. She had a history of type 2 diabetes and arterial hypertension, untreated recurrent urinary tract infections and cesarean surgery. During physical examination, the patient was sleepy and lethargic, had dehydrated oral mucosa, tachycardia with respiratory distress, ballooning abdomen at the expense of abundant fatty panniculus, presence of a non-incarcerated, reducible umbilical hernia, percussion with generalized tympany, palpation without presence of hepatic or splenomegaly and without peritoneal irritation signs. Left Giordano sign was positive and right was negative, ureteral points were negative. Vital signs during her admission showed blood pressure of 90/50 mmHg, cardiac frequency 105, breathing frequency 24, oxygen saturation 90% with a pulse oximeter, capilar glycemia 246 mg/dL. Upon admission, labs reported leukocytosis 40.43×103/uL at the expense of neutrophilia of 39.06×103/uL, hemoglobin 7.4 g/dL, hematocrit 25.6%, platelets 831×103/uL, venous central glycemia 173 mg/dL, creatinine 3.73 mg/dL, urea 96 mg/dL, urea nitrogen 45 mg/dL, uric acid 12.7 mg/dL, prothrombin time (PT) 18.4 seconds, international normalized ratio (INR) 1.33, partial thromboplastin time (PTT) 27.2 seconds, arterial blood gases with pH 7.22, pCO2 35.3 mmHg, pO2 49.2 mmHg, lactate 1.6 mmol/L, oxygen saturation 78.3%, HCO3 14 mmol/L, Base excess –12.5 mmol/L, potassium 4.5 mmol/L, sodium 136 mmol/L; General urine test with cloudy yellow urine, glycosuria of 250 mg/dL, proteinuria of 100 mg/dL, hemoglobinuria of 80 mg/dL, ketones were negative and urine microscope examination with leukocytes 45–50/field, erythrocytes 12–14/field, scant cells, moderate amorphous urate crystals, and moderate bacteria. A urine culture was also requested, with subsequent negative results.
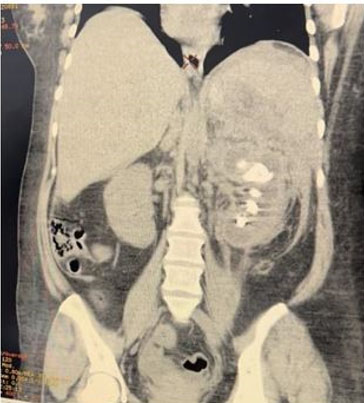
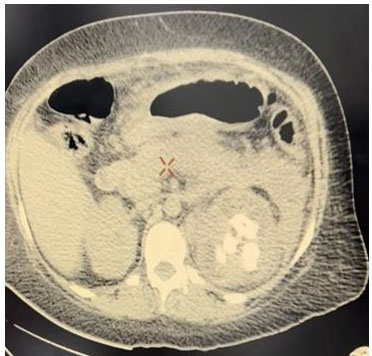
A simple abdomen CT scan was performed, finding an enlarged left kidney, with loss of morphology, striation of perirenal fat, thickening of Gerota’s fascia, dilation of minor calyces, presence of a hyperdense image on the large central portion compatible with staghorn stone and hypodense dots in upper left kidney pole compatible with gas in the collecting system (Figure 1 and Figure 2). Right kidney was with preserved dimension and morphology.
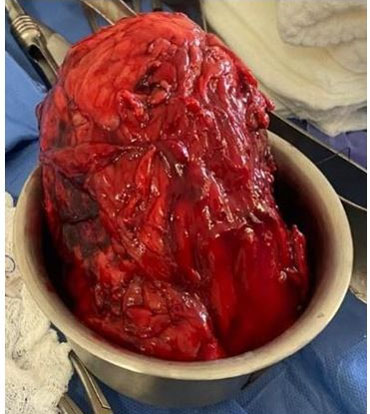
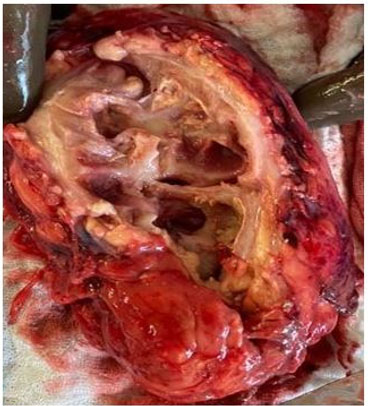
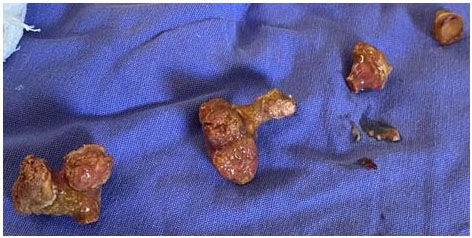
The patient was stabilized, joint management was performed with the nephrology service and fluid resuscitation treatment, administration of norepinephrine and broad-spectrum antibiotics were started, 4 packed erythrocytes were transfused, improving general conditions for four days with conservative management and transfer to the operating room performing simple nephrectomy by open lumbotomy approach, finding infiltration of perirenal fat and Gerota’s fascia (Figure 3, Figure 4, Figure 5). She got admitted to the intensive care unit, being extubated and under management of norepinephrine managing to wean off norepinephrine over the next 72 hours and maintaining vital signs in normal parameters, diuresis 1300–1800 mL in 24 hours with a urinary index of 0.6–0.83 mL/kg/h, eventually getting discharged and sent to hospitalization.
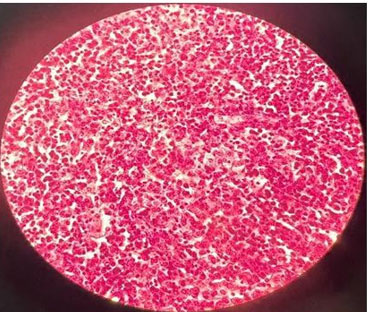
Control laboratories were taken, finding improvement in laboratory parameters: leukocytes 16.2×103/uL, neutrophils 14.3×103/uL, platelets 418×103/uL, glucose 117 mg/dL, creatinine 0.62 mg/dL, urea 24 mg/dL, potassium 3.9 mmol/L, sodium 136 mmol/L, in terms of clinical conditions, patient alertness, oriented, vital signs with blood pressure of 118/58 mmHg, heart rate of 96 beats/minute, respiratory rate of 18/minute, saturating 95% without supplemental oxygen, capillary glycemia of 132 mg/dL, diuresis quantified in 1700 mL/24 hours with urine index output of 0.78 mL/kg/h, for which it is decided that she was discharged from the hospital for improvement, specifying a total of 15 days of hospital stay such as intravenous antibiotic therapy. Subsequently, a histopathological report was obtained, resulting in xanthogranulomatous pyelonephritis with scar fibrosis, glomerulosclerosis, without evidence of malignancy (Figure 6).
Discussion
Within the xanthogranulomatous pyelonephritis, it has been classified into three stages according to Malek’s classification: Stage I or focal: confined to the kidney; stage II or diffuse: there is infiltration into Gerota’s capsule; and stage III or extrarenal: it extends to the perinephric space and retroperitoneal structures [11],[12], in our case a stage II was found, confirming that 90% cases present a diffuse form and only 10% present a localized form [2],[7]. Early diagnosis plays a crucial role in reducing the morbidity and mortality of xanthogranulomatous pyelonephritis.
The challenge of preoperative diagnosis in this pathology is high, since it is confirmed by histopathological results and imaging studies may be suggestive of some neoplastic pathology, both renal and retroperitoneal, often allowing it to be differentiated during the trans-surgical event [1],[2],[3],[7].
In most cases, it is useful to apply an initial antibiotic treatment, after which a surgical intervention is performed [3]. When planning surgical intervention for this condition, the need for massive blood transfusion, visceral injury, and prolonged operation time must be taken into account. Always having a multidisciplinary approach through a surgical (urology and general surgery) and medical (anesthesia, intensive care, nephrology, diabetes service, and infectious diseases) team is a vital step in managing these difficult cases [10]. After surgical intervention, clinical and biochemical improvement is progressive with a high discharge rate due to improvement [13].
In the Australian series by Addison et al., 35 cases of xanthogranulomatous pyelonephritis were collected, where 68% (n = 24) of the patients experienced postoperative complications. 5% of the patients died within 30 days, as well as 32% suffered a significant postoperative complication [10].
In a small Mexican series by Torres et al., 171 probable cases were collected, where only 18 of them (10.5%) were diagnosed with xanthogranulomatous pyelonephritis, but only 10% were associated with mortality [13].
The systematic review by Harley et al. is the largest to date, reporting 1139 patients with xanthogranulomatous pyelonephritis compiled in the last 21 years from 40 international studies reported in Cochrane, Embase, and Medline, which refers to the classic perioperative mortality. Of 40%, contrasting with his study, arguing that 630 patients referred from 27 studies, 18 of them died a year after their diagnosis, concluding that currently patients with xanthogranulomatous pyelonephritis have a low mortality, contrasting with what has historically been reported [6], leaving the gap open for new publications on mortality and morbidity in each country, being important to correctly document each case of xanthogranulomatous pyelonephritis.
Conclusion
Xanthogranulomatous pyelonephritis is the result of a chronic inflammatory process, where the renal parenchyma progressively loses functionality as it is replaced by lipid-laden macrophages and fibrotic tissue. In this case, the patient was admitted with septic shock, preoperative management was key on reducing perioperative morbidity and mortality, later, when performing the nephrectomy, she had a clear improvement in the following days, until the condition completely remitted. Statistically, we lack current information on mortality and postoperative complications, leaving this work as a possibility for new cases to be described and their subsequent registration.
REFERENCES
1.
2.
Yi M, Liu Y, Chen Q. Xanthogranulomatous pyelonephritis with polycystic kidney disease as a mimic of cystic renal cell carcinoma: A case report. BMC Urol 2023;23(1):58. [CrossRef]
[Pubmed]

3.
Barboza MP, Nottingham CU, Calaway AC, et al. Xanthogranulomatous pyelonephritis: A comparison of open and minimally-invasive surgical approaches. J Robot Surg 2021;15(4):611–7. [CrossRef]
[Pubmed]

4.
5.
Gravestock P, Moore L, Harding C, Veeratterapillay R. Xanthogranulomatous pyelonephritis: A review and meta-analysis with a focus on management. Int Urol Nephrol 2022;54(10):2445–56. [CrossRef]
[Pubmed]

6.
Harley F, Wei G, O’Callaghan M, Wong LM, Hennessey D, Kinnear N. Xanthogranulomatous pyelonephritis: A systematic review of treatment and mortality in more than 1000 cases. BJU Int 2023;131(4):395–407. [CrossRef]
[Pubmed]

7.
Ciccarese F, Brandi N, Corcioni B, Golfieri R, Gaudiano C. Complicated pyelonephritis associated with chronic renal stone disease. Radiol Med 2021;126(4):505–16. [CrossRef]
[Pubmed]

8.
Kundu R, Baliyan A, Dhingra H, Bhalla V, Punia RS. Clinicopathological spectrum of xanthogranulomatous pyelonephritis. Indian J Nephrol 2019;29(2):111–5. [CrossRef]
[Pubmed]

9.
10.
Addison B, Zargar H, Lilic N, Merrilees D, Rice M. Analysis of 35 cases of xanthogranulomatous pyelonephritis. ANZ J Surg 2015;85(3):150–3. [CrossRef]
[Pubmed]

11.
Jang TL, McKoy T, Hakim J, Polenakovik HM. Xanthogranulomatous pyelonephritis – A diagnostic and therapeutic dilemma. Am J Med Sci 2023;365(3):294–301. [CrossRef]
[Pubmed]

12.
Kisa E, Keskin MZ, Yucel C, Yalbuzdag ON, Karabıcak M, Ilbey YO. Effect of kidney volume on the results of nephrectomy performed for xanthogranulomatous pyelonephritis. Cureus 2019;11(1):e3976. [CrossRef]
[Pubmed]

13.
Torres-Gómez J, Martinez-Alonso IA, Campos-Salcedo JG, et al. Reporte de 18 casos de pielonefritis xantogranulomatosa. Rev Mex Urol 2015;75(4):187–90. [CrossRef]

SUPPORTING INFORMATION
Acknowledgments
We thank the staff of the “Hospital General de Ciudad Juarez” for their support and constant drive to learn.
Author ContributionsCesar Alberto Lopez Jaime - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Armando Alonso Morales - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Carlos Tadeo Perzabal Avilez - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Francisco Netzahualc Hernandez Garza - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Cesar Alberto Lopez Jaime et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.