|
Clinical Image
Systemic amyloidosis causing profound retroperitoneal and perinephric calcified deposits
1 Virginia Tech Carilion School of Medicine, Roanoke, VA, USA
2 University of Virginia Medical School, Charlottesville, VA, USA
3 Urology, University of Virginia Medical School, Charlottesville, VA, USA
Address correspondence to:
Jacob Hartman-Kenzler
2 Riverside Circle, Roanoke, VA 24016,
USA
Message to Corresponding Author
Article ID: 100022Z15JK2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Hartman-Kenzler J, Salomon M, Yeaman C, Krupski T. Systemic amyloidosis causing profound retroperitoneal and perinephric calcified deposits. J Case Rep Images Urol 2021;6:100022Z15JK2021.ABSTRACT
No Abstract
Keywords: Amyloidosis, Lymphoplasmacytic lymphoma, Retroperitoneal calcifications, Systemic amyloidosis
Case Report
A 66-year-old man presented in the emergency department with a three-day history of lethargy, poor oral intake, decreased urine output, lower abdominal pain, and nausea. The patient had a history of lymphoplasmacytic lymphoma (LPL) with retroperitoneal amyloid deposition in remission after autologous peripheral blood stem cell transplant. Other comorbidities included type 2 diabetes mellitus, hypertension, macular degeneration, peripheral neuropathy, and chronic kidney disease (CKD) stage IV [creatinine (Cr) 2.5, glomerular filtration rate (GFR) 26] secondary to amyloidosis and hydronephrosis status post bilateral ureteral stent placement and subsequent removal one year prior, which was complicated by subsequent refractory bleeding and urinary tract infection (UTI). Prior surgical history was colonoscopy, stent placement, and cardiac stress test. Notable laboratory abnormalities on presentation included hyperkalemia, uremia, elevated creatinine, and elevated alkaline phosphatase [Na 125, K 6.3, blood urea nitrogen (BUN) 100, Cr 5.0, GFR 11, alkaline phosphatase (ALP) 431].
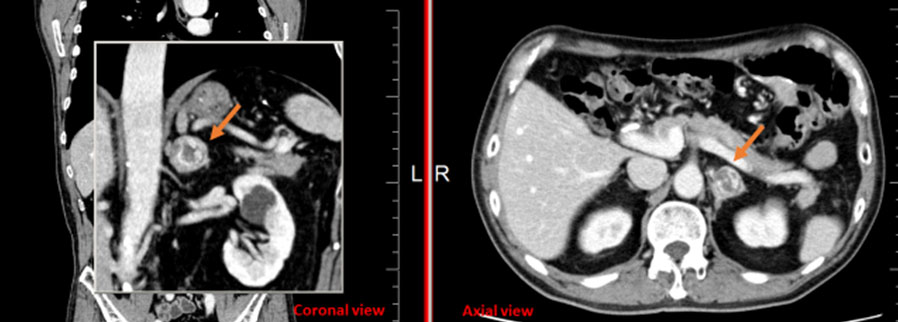
During admission, stage IV acute-on-chronic kidney injury did not respond to diuresis. Medical management of hyperkalemia included gentle diuresis as well as temporizing measures of dextrose, insulin, and calcium gluconate. Management of hyponatremia was accomplished with intravenous (IV) fluid administration and frequent laboratory checks to avoid overcorrection. Urology was consulted on hospital day 3. Based on previous ureteroscopy showing narrowed right ureter, there was concern for ureteral obstruction contributing to kidney injury compounded by massive prostatomegaly and narrow bladder neck. The patient and family were presented with treatment options including observation, ureteral stent placement, and interventional radiology directed percutaneous nephrostomy or nephroureteral stent placement. After consideration, the patient elected to move forward with nephroureteral stent placement on hospital day 19 to address hydronephrosis and UTI as well as refractory acute kidney injury (AKI) on CKD (Cr 3.0, GFR 21). Left nephroureteral stent placement was successful; however, the right nephroureteral stent was unable to be placed due to ureteral narrowing. Right percutaneous nephrostomy tube was placed. Subsequently, the patient experienced continued oliguria and worsened gross hematuria. A computed tomography (CT) scan of the abdomen and pelvis was performed to rule out retroperitoneal hemorrhage and confirm placement of the stent and tube.
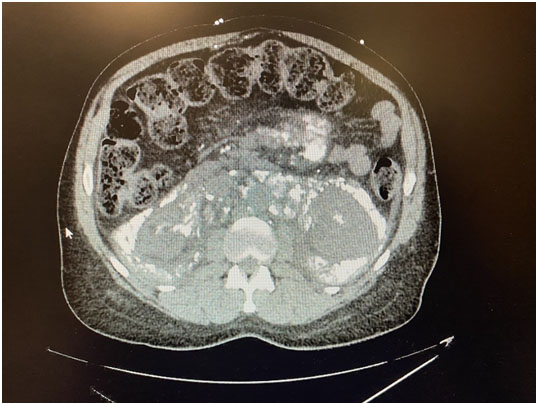
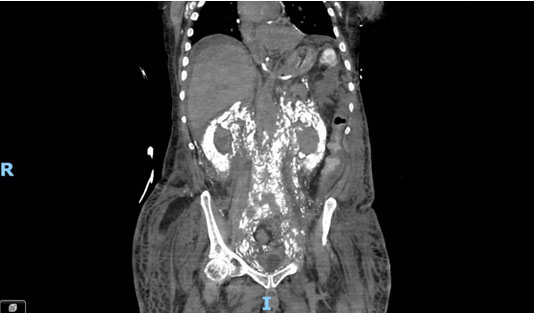
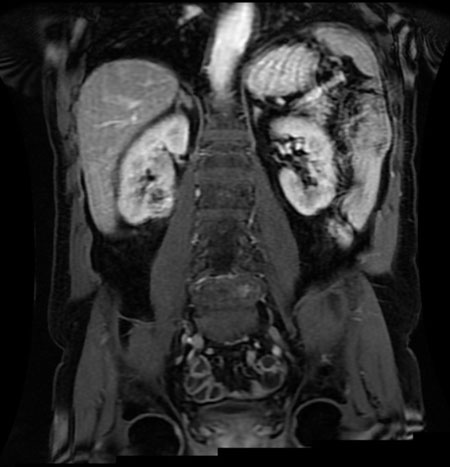
Computed tomography imaging (Figure 1 and Figure 2) revealed extensive systemic amyloidosis, progressing from previous scans with notable deposition surrounding the renal parenchyma and throughout the retroperitoneum. There were also prominent amyloid deposits along the path of the ureters, infiltrating the ureteral tissue itself, as well as periureteral tissue, and nearby lymphatics. This deposition of amyloid contributed to the patients AKI through chronic hydronephrosis and likely caused intrinsic kidney injury through amyloid deposition in the renal parenchyma, which may have been compounded by some element of obstruction from diffuse retroperitoneal amyloid deposition. The patient was managed with continuous renal replacement therapy, but ultimately elected for hospice care and passed due to renal failure.
Discussion
Systemic amyloidosis is an uncommon disease that is very rarely associated with LPL [1]. Generally, when amyloid accumulates in the urinary tract it is a localized amyloidosis that occurs mainly in the urinary bladder, and very rarely in the kidneys [2]. While there are sparse case reports of systemic amyloidosis invasion of various retroperitoneal structures [3], this is the first case report, to our knowledge, of diffuse retroperitoneal amyloid deposition secondary to LPL and this level of urinary tract involvement.
Patients with amyloidosis secondary to LPL have been documented to present with fatigue, weight loss, macroglossia, nephrotic syndrome, peripheral neuropathy, and compressive syndromes secondary to large deposits of amyloid in lymph nodes and organ viscera [4]. However these presentations are often due to focal amyloidosis and do not necessarily occur together. Therefore, it is important to note our patient’s unique presentation and constellation of symptoms, including lethargy, poor oral intake, decreased urine output, lower abdominal pain, nausea, hematuria, hydronephrosis, and UTI. The severe extent of amyloid deposits within our patient’s kidneys, along the ureters, and in the bladder, likely account for the hematuria and other urologic symptoms on presentation. Furthermore, ureteral obstruction secondary to amyloidosis likely contributed to our patient’s history of UTIs.
Typically, when amyloid deposits in the kidneys, the primary urological presentation is nephrotic syndrome with characteristic focal findings on CT [5]. Previous imaging reports describe these urinary symptoms presenting with much earlier stages of amyloidosis, usually before such extensive calcification [6]. It is important to establish a chronology for these unique cases in the context of this patient’s impressive imaging to inform future clinical decision making and follow-up practices.
Conclusion
Extensive invasion of the retroperitoneum and urinary tract are uncommon findings in systemic amyloidosis and can be associated with lymphoplasmacytic lymphoma, though uncommon. Our patient’s clinical findings and imaging highlight an impressive and unique presentation of this condition that has little previous documentation.
REFERENCES
1.
Wechalekar AD, Chakraborty R, Lentzsch S. Systemic amyloidosis due to low-grade lymphoma. Hematol Oncol Clin North Am 2020;34(6):1027–39. [CrossRef]
[Pubmed]

2.
Kawashima A, Alleman WG, Takahashi N, Kim B, King BF Jr, LeRoy AJ. Imaging evaluation of amyloidosis of the urinary tract and retroperitoneum. Radiographics 2011;31(6):1569–82. [CrossRef]
[Pubmed]

3.
Glynn TP Jr, Kreipke DL, Irons JM. Amyloidosis: Diffuse involvement of the retroperitoneum. Radiology 1989;170(3 Pt 1):726. [CrossRef]
[Pubmed]

4.
Cohen AD, Zhou P, Xiao Q, et al. Systemic AL amyloidosis due to non-Hodgkin’s lymphoma: An unusual clinicopathologic association. Br J Haematol 2004;124(3):309–14. [CrossRef]
[Pubmed]

5.
Georgiades CS, Neyman EG, Barish MA, Fishman EK. Amyloidosis: Review and CT manifestations. Radiographics 2004;24(2):405–16. [CrossRef]
[Pubmed]

6.
Urban BA, Fishman EK, Goldman SM, et al. CT evaluation of amyloidosis: Spectrum of disease. Radiographics 1993;13(6):1295–308. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Jacob Hartman-Kenzler - Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Michael Salomon - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Clinton Yeaman - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tracey Krupski - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Jacob Hartman-Kenzler et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.