|
Case Report
Ipsilateral synchronous renal cell carcinoma and transitional cell carcinoma of the pelvicalyceal system
1 Department of Urology, University of Balamand, Saint George Hospital University Medical Hospital, Beirut, Lebanon
2 Department of Internal Medicine, University of Balamand, Saint George Hospital University Medical Hospital, Beirut, Lebanon
3 American University of Beirut, Faculty of Arts and Sciences, Beirut, Lebanon
4 Department of Medical Imaging, University of Balamand, Saint George Hospital University Medical Hospital, Beirut, Lebanon
Address correspondence to:
Teddy Jabbour
MD, Department of Urology, University of Balamand, Saint George Hospital University Medical Hospital, Beirut,
Lebanon
Message to Corresponding Author
Article ID: 100021Z15TJ2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Jabbour T, Akl B, Haydar A, Jabbour C, Haddad L, Jabbour M. Ipsilateral synchronous renal cell carcinoma and transitional cell carcinoma of the pelvicalyceal system. J Case Rep Images Urol 2021;6:100021Z15TJ2021.ABSTRACT
Introduction: Ipsilateral synchronous renal cell carcinoma and transitional cell carcinoma of the 8 pelvicalyceal system are rare occurrences with data from 1977 showing a 0.14% prevalence with a 24% association with smoking. 90% of patients present with hematuria. Serum hepcidin levels and serum GDF-15 levels are typically elevated in patients with upper urinary tract transitional cell carcinoma (TCC) and renal cell carcinoma (RCC). Possible associations with genomic mutations such as in Birt-Hogg Dubé syndrome, due to a mutation in the folliculin (FLCN) gene, and in Lynch syndrome, due to a mutation in four different mismatch repair genes. The current recommended treatment is nephroureterectomy. Due to the rarity of this disease, prospective studies regarding the usage of neoadjuvant chemotherapy treatment have been limited.
Case Report: We present the rare case of a 72-year-old male found to have Ipsilateral synchronous renal cell carcinoma and transitional cell carcinoma of the pelvicalyceal system that underwent a laparoscopic nephroureterectomy that showed typical findings of a Stage pT1a and WHO/ISUP grade 2 RCC and a Stage pTa TCC in the renal pelvis, with a stage pTa and Grade I in the bladder.
Conclusion: Further follow-up reports must be provided on this rare entity with long-term follow-up to provide further recommendation for the treatment of synchronous ipsilateral TCC and RCC.
Keywords: Ipsilateral, Renal cell carcinoma, Synchronous, Transitional cell carcinoma
Introduction
Around 50 cases of ipsilateral synchronous renal cell carcinoma (RCC) and transitional cell carcinoma (TCC) of the renal pelvis were reported in the available literature showing the rarity of this synchronous presentation [1],[2],[3],[4]. Only two possible etiologies have been described explaining the decreased prevalence of this disease. The first etiology posed in 1990 in a Bulgarian study by Stratev highlighted that 31% of tumors in the renal pelvis remain undetected, while 50% of cases of tumors in the ureter are typically unrecognized [5]. The second etiology recently explained on 2009 by Tsai explained that since both RCC and TCC are associated with inflammatory disease of the kidney, this renders imaging modalities difficult to detect their presence and to be mistaken for pyelonephritis should there no structural findings be found [6].
In the context of the paucity of available literature tackling this presentation, we present the case of a 72-year-old male patient with ipsilateral synchronous RCC and TCC of the upper tract.
Case Report
A 72-year-old male patient, non-smoker, previously healthy, presented with a chief complaint of painless gross hematuria of one month duration. He reported no flank pain or discomfort and a negative family history for renal pathology. An ultrasound of the bladder and upper tract was performed showing the presence of a right, well-defined, cortical isoechoic mass in the lower pole of the right kidney measuring approximately 3 cm in size; in addition to thickening of the bladder wall.
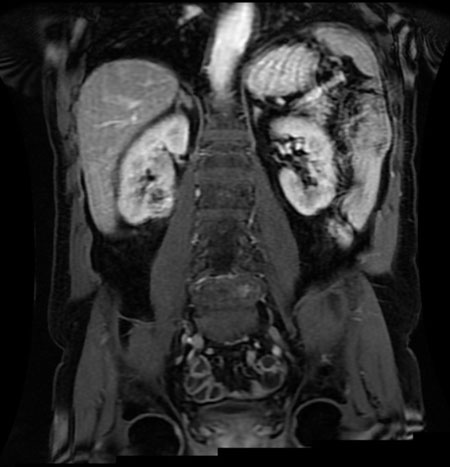
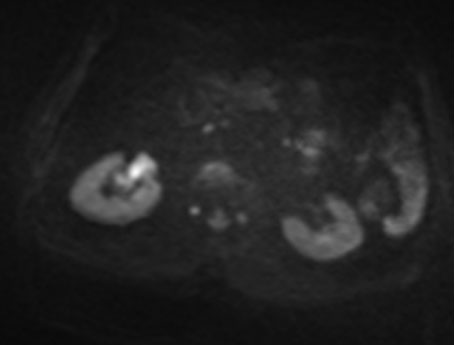
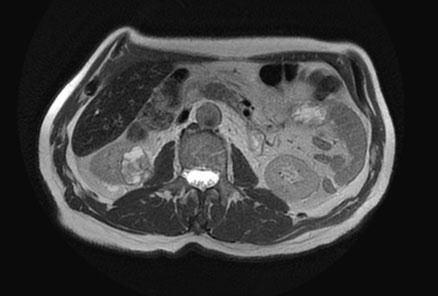
Renal magnetic resonance imaging (MRI) was performed for better characterization of the mass and showed a 3.8×2.2×3.2 cm exogenous lower pole right renal lesion with multiple thickened septations, and a soft tissue component showing heterogeneous signal on T2 images suggestive of RCC. An additional right renal pelvic mass measuring up to 2 cm in the transverse axis was found, neoplastic in nature, suggestive of TCC (Figure 1, Figure 2, Figure 3).
The patient was therefore scheduled for cystourethroscopy and retrograde pyelography of the right upper tract.
Upon admission, the patient had a negative review of systems and an unremarkable physical examination.
Cystourethroscopy demonstrated a bladder neck tumor that was subsequently resected. Retrograde pyelography of the right collecting system demonstrated the presence of a right pelvic mass. Wash cytology from the upper tract was taken and sent for analysis.
Pathology results showed a papillary transitional cell tumor of the bladder invading the lamina propria stage pT1 grade III. Right upper tract cytology showed the presence of high-grade tumor cells. The patient’s hospital stay was smooth and uncomplicated.
Six weeks following the TURBT, the patient presented back for a re-TURBT and a right laparoscopic nephroureterectomy. The patient’s hospital stay was uncomplicated. He was discharged on day four postoperative day and given appropriate recommendations for follow-up.
The pathology results of the specimens taken showed a low grade papillary noninvasive urothelial carcinoma: stage pTa and grade I at the site of the previous bladder resection. The renal mass was shown to be a clear cell renal carcinoma of the lower pole measuring 3 cm in the greatest dimension, and limited to the kidney: Stage pT1a and WHO/ISUP grade 2. There was no extension of the tumor to the renal sinus fat or to the perinephric fat or the renal vein. There was no evidence of rhabdoid or sarcomatoid features, tumor necrosis, or of neoplastic lymphovascular invasion.
In addition, a high grade papillary noninvasive urothelial carcinoma of the renal pelvis was found, with focal squamous differentiation, extending to the middle and lower calyces, and measuring 5 cm in the greatest dimension, without invasion of the renal parenchyma or of the renal sinus fat: Stage pTa.
A low grade noninvasive papillary urothelial carcinoma of the ureter, in a background of diffuse florid Von Brunn nests (numerous urothelial nests arranged in a lobular non-infiltrating pattern in the lamina propria) was noted. The distal ureteral margin or bladder cuff was negative for urothelial carcinoma in situ and for invasive urothelial carcinoma.
Discussion
Renal cell carcinoma accounts for approximately 90% of all renal malignancies and for 3% of adult cancers. There are several types of renal carcinomas of different prevalence patterns: clear cell RCC representing 70% of RCCs, papillary being 10–15%, chromophobe 5%, and collecting duct tumors with an even lower prevalence [7]. On the other hand, TCC of the renal pelvis or ureter represents 5–7% of all urinary tract tumors while accounting for less than 1% of genitourinary neoplasms [8]. As for their synchronous and ipsilateral presence, there are no up-to-date statistics on its prevalence. Von Eschenbach et al. reported a prevalence of 0.14% for ipsilateral synchronous RCC and TCC of the renal pelvis out of a total of 700 nephrectomies done [9].
In 2005, 47 cases of synchronous renal and transitional cell carcinomas were reported. It was noted that 24% of patients were found to be smokers, but no specific risk factors were identified. In addition, this Spanish review found that the typical presentations of synchronous RCC and TCC were that of RCC and TCC independently; hematuria was found in 90% of the cases, flank pain was present in 19% of cases, and a palpable flank mass was evident in 14% of cases [4].
Possible syndromic associations include the Birt-Hogg-Dubé syndrome (BHD). Birt-Hogg-Dubé syndrome is a rare malignancy susceptibility syndrome caused by a mutation in the folliculin FLCN gene [10]. This syndrome is characterized by renal cell tumors, pulmonary, and dermatological manifestations [10]. Given the lack of associated symptoms and family history, BHD was excluded from the differential diagnosis. On the other hand, a retrospective study showed that 28% of patients with Lynch syndrome manifested upper tract urothelial carcinoma (UTUC) [11]. Lynch syndrome is an autosomal dominant syndrome due to a mutation in four different mismatch repair genes [11]. This syndrome should be suspected in patients younger than 60 years of age presenting with UTUC and/or meeting the “3-2-1” rule of Lynch syndrome [12]. Since this patient is above 60 years old and lacks any relevant familial history, this diagnosis was not further investigated.
Regarding upper tract urinary cytology and its usage for detecting upper tract neoplasms/atypical cells, a review of the literature between the years of 2011 and 2014 showed a sensitivity of 64–74% [13],[14],[15],[16],[17], a specificity of around 50%, and a positive predictive value of 98% [17]. One of the limitations of these studies was that the grading of urine cytology wasn’t performed systematically; in addition, there is no association to accurately predict the degree of invasiveness or the grade of the upper tract pathology with the cytology [14],[17] preventing a deduction of the correlation between the grading on histopathology and a positive upper tract urinary cytology [17]. However, a positive cytology was found in 74.5% and 73% of high-grade and low-grade tumors, respectively, showing the clinical utility of upper tract urinary cytology in detecting neoplastic cells [17].
In 2019, Traeger et al. designed a retrospective study on patients diagnosed with upper tract TCC and RCC. It concluded that serum hepcidin levels and serum GDF-15 levels were found to be elevated in these patients and were associated with decreased survival rates and higher rates of relapse and metastasis. This showed that hepcidin and GDF-15 can be used as a prognostic indicator for such patients. Even though this study is a small cohort study, it shows statistically significant outcomes that warrant larger cohort studies to confirm it [18].
The adopted surgical protocol for RCC is nephrectomy, radical or partial, depending on the characteristics of the tumor [19]. As for upper tract TCC of the kidney, the recurrence rate in the ipsilateral ureteral stump following endoscopic management is reported to be 30–70%, with high grade recurrences in the ureteral stump being associated with poorer prognosis [20]. Hence, nephroureterectomy with a wide bladder cuff represents the main line of treatment for localized high-risk disease.
Overall, the prognosis of synchronous TCC and RCC of the ipsilateral kidney is inconclusive. Based on the review by Arjona et al. (2005) of synchronous renal tumors, the simultaneous occurrence does not worsen the prognosis [7]. Contrarily, Lu et al. found that 24% had metastasis at initial examination, and 34% had bladder neoplasms present concluding the tendency of simultaneous occurrence to exhibit poorer prognosis [19]. However, Dutta et al. reported that the more aggressive of the two tumors is the determinant of prognosis [5].
The European Association of Urology 2021 Guidelines on upper tract urothelial cancer state that, in high-risk patients, neoadjuvant chemotherapy has been associated with significant downstaging at surgery and ultimately survival benefit compared to radical nephroureterectomy (RNU) alone [21]. Due to the rarity of simultaneous and ipsilateral occurrence, attempted prospective trials have been limited [22].
In 2010, the American Cancer Society reported that the prevalence of genitourinary tumors has increased by approximately 10% [23]. All recent studies refer to the 1977 study on 700 nephrectomies conducted by Von Eschenbach et al., to highlight the prevalence and rarity of this disease. This calls for further research on the prevalence of TCC in the renal pelvis with synchronous RCC specifically as there has not been any recent study tackling this simultaneous occurrence. Lastly, definitive recommendations regarding the treatment of ipsilateral synchronous RCC and TCC of the kidney based on prospective studies are required to further characterize the disease.
Conclusion
Ipsilateral synchronous RCC and TCC of the kidney are rare entities with possible genetic associations and no accurate prevalence. The prognosis of the disease is currently a matter of debate as no conclusive data has been determined. There are no definite treatment guidelines as more prospective studies are needed to determine the benefit of the currently recommended treatment regimens.
REFERENCES
1.
Atilgan D, Uluocak N, Parlaktas BS. Renal cell carcinoma of the kidney with synchronous ipsilateral transitional cell carcinoma of the renal pelvis. Case Rep Urol 2013;2013:194127. [CrossRef]
[Pubmed]

2.
Dutta G, Silver D, Oliff A, Harrison A. Synchronous renal malignancy presenting as recurrent urinary tract infections. Case Rep Urol 2011;2011:832673. [CrossRef]
[Pubmed]

3.
Leveridge M, Isotalo PA, Boag AH, Kawakami J. Synchronous ipsilateral renal cell carcinoma and urothelial carcinoma of the renal pelvis. Can Urol Assoc J 2009;3(1):64–6. [CrossRef]
[Pubmed]

4.
Arjona MF, Arrontes DS, Barbosa FDC, Morillas FB, Aranguez IC, González L. Synchronous renal clear-cell carcinoma and ipsilateral transitional-cell carcinoma: Case report and bibliographic review. [Article in Spanish]. Arch Esp Urol 2005;58(5):460–3. [CrossRef]
[Pubmed]

5.
Stratev S. The diagnostic difficulties in multiple tumors of the urothelium. Khirurgiia (Sofiia) 1990;43(4):32–5.
[Pubmed]

6.
Tsai TH, Tang SH, Chuang FP, et al. Ipsilateral synchronous neoplasms of kidney presenting as acute pyelonephritis and bladder metastasis. Urology 2009;73(5):1163.e9–11. [CrossRef]
[Pubmed]

7.
Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol 2009;16(5):432–43. [CrossRef]
[Pubmed]

8.
Melamed MR, Reuter VE. Pathology and staging of urothelial tumors of the kidney and ureter. Urol Clin North Am 1993;20(2):333–47.
[Pubmed]

9.
Voneschenbach AV, Johnson DE, Ayala AG. Simultaneous occurrence of renal adenocarcinoma and transitional cell carcinoma of the renal pelvis. J Urol 1977;118(1 Part 1):105–6. [CrossRef]
[Pubmed]

10.
Benusiglio PR, Giraud S, Deveaux S, et al. Renal cell tumour characteristics in patients with the Birt-Hogg-Dubé cancer susceptibility syndrome: A retrospective, multicentre study. Orphanet J Rare Dis 2014;9:163. [CrossRef]
[Pubmed]

11.
Mork M, Hubosky SG, Rouprêt M, et al. Lynch syndrome: A primer for urologists and panel recommendations. J Urol 2015;194(1):21–9. [CrossRef]
[Pubmed]

12.
Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet 2014;7:147–58. [CrossRef]
[Pubmed]

13.
Jovanovic M, Soldatovic I, Janjic A, et al. Diagnostic value of the nuclear matrix protein 22 test and urine cytology in upper tract urothelial tumors. Urol Int 2011;87(2):134–7. [CrossRef]
[Pubmed]

14.
Messer J, Shariat SF, Brien JC, et al. Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int 2011;108(5):701–5. [CrossRef]
[Pubmed]

15.
Straub J, Strittmatter F, Karl A, Stief CG, Tritschler S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. In Urol Oncol 2013;31(7):1166–70. [CrossRef]
[Pubmed]

16.
Xu C, Zeng Q, Hou J, et al. Utility of a modality combining FISH and cytology in upper tract urothelial carcinoma detection in voided urine samples of Chinese patients. Urology 2011;77(3):636–41. [CrossRef]
[Pubmed]

17.
Sverrisson EF, Kim T, Espiritu PN, et al. The merits of cytology in the workup for upper tract urothelial carcinoma – a contemporary review of a perplexing issue. Int Braz J Urol 2014;40(4):493–8. [CrossRef]
[Pubmed]

18.
Traeger L, Ellermann I, Wiethoff H, et al. Serum Hepcidin and GDF-15 levels as prognostic markers in urothelial carcinoma of the upper urinary tract and renal cell carcinoma. BMC Cancer 2019;19(1):74. [CrossRef]
[Pubmed]

19.
Lu Q, Zhuang J, Guo H. Renal cell carcinoma with synchronous ipsilateral urothelial carcinoma of the renal pelvis. Oncol Lett 2017;13(6):4521–5. [CrossRef]
[Pubmed]

20.
Murphy DM, Zincke H, Furlow WL. Management of high grade transitional cell cancer of the upper urinary tract. J Urol 1981;125(1):25–9. [CrossRef]
[Pubmed]

21.
Rouprêt M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 2011;59(4):584–94. [CrossRef]
[Pubmed]

22.
Kubota Y, Hatakeyama S, Tanaka T, et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: A multicenter study. Oncotarget 2017;8(60):101500–8. [CrossRef]
[Pubmed]

23.
Mucciardi G, Galì A, D’Amico C, Muscarà G, Barresi V, Magno C. Transitional cell carcinoma of the renal pelvis with synchronous ipsilateral papillary renal cell carcinoma: Case report and review. Urol Case Rep 2015;3(4):93–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Teddy Jabbour - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bernard Akl - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Asad Haydar - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christelle Jabbour - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Laura Haddad - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Michel Jabbour - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Teddy Jabbour et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.